SEATTLE--(BUSINESS WIRE)--Aug 1, 2023--
Dr. Ching-Hon Pui, Co-Leader, Hematological Malignancies Program, St. Jude, Memphis, TN and 2020 American Cancer Society Medal of Honor Awardee, “I am very impressed with your previous efforts to help my former patient. You provided AVM0703 in a record time (less than 24 hours) to my patient by sending your colleague by plane to hand deliver the medication to St. Jude from Seattle, WA. The drug yielded an amazing response. Even though my patient had very advanced leukemia, AVM0703 helped to control the disease and bridged him to CAR T cells therapy.”
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230731194052/en/
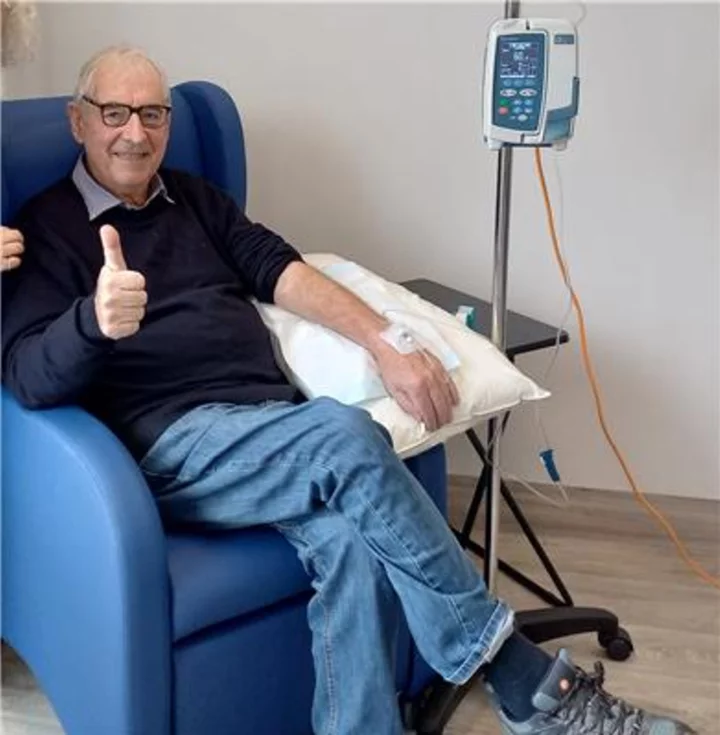
A metastatic esophageal adenocarcinoma patient during his 8th AVM0703 infusion. "Overall I have to say AVM0703 has been the only drug used to tackle my throat cancer since my first AVM0703 treatment on Nov 18th Nov 2022. The result has been rewarding with little by way of side effects apart from dull pain emerging 24 hours after treatment periods round cancer infected upper chest area. This pain seems to last approx 3 hours then fades away and sometimes repeats again on the second day after treatment. No doubt this pain is AVM0703 at work, targeting the cancer. Thank you all for your advice and guidance, by putting the wellness back into my health again. Thank you to all at AVM Biotechnology." (Photo: Business Wire)
AVM Biotechnology, a clinical stage company actively enrolling Phase 2 for Relapsed/Refractory Non-Hodgkin’s Lymphoma of all subtypes (partially funded by NCI Ph II FastTrak grant 1R44CA272096), today announced that twenty-eight (28) solid tumor and blood cancer patients have been treated with its immunomodulatory drug AVM0703 through Expanded Access (EAP)/Compassionate Use (CUP) programs. Cancers that have been treated include highly relapsed/refractory, some imminently terminal, patients with glioblastoma, metastatic breast cancer (two with advanced bone metastases), metastatic ovarian cancer, metastatic gastric cancer, Hodgkin’s Lymphoma, mixed phenotype acute leukemia, B-ALL, metastatic colon cancer, malignant myxoid spindle cell neoplasm, non-small cell lung cancer, DLBCL with CNS involvement, desmoplastic small round cell tumor, metastatic esophageal adenocarcinoma, prostate cancer, anaplastic T-cell Non-Hodgkin’s Lymphoma and inoperable/chemotherapy ineligible CNS squamous cell carcinoma.
Immunomodulatory AVM0703's relatively broad anti-cancer activity is hypothesized to be due to mobilization of a highly active gamma/delta T-cell receptor expressing immune cell, which is programmed to recognize special stress signals produced by most cancer cells but not normal cells. Requests for additional information about immunomodulatory AVM0703 and its relatively broad activity against solid tumors and blood cancers can be made by contacting compassionateuse@avmbiotech.com.
“AVM Biotechnology is committed to providing AVM0703 to patients who cannot participate in our enrolling clinical trial. Based on absence of safety concerns and responses reported to date, we believe AVM0703 may provide benefits to all cancer patients who are desperately searching for options. Our team at AVM Biotechnology is dedicated to providing hope to patients and their loved ones.” Theresa Deisher, AVM Biotechnology, Founder and CEO.
Requests for Expanded Access in the US must be made by a US licensed physician. Physicians can learn more about the AVM0703 EAP on clinicaltrials.gov and can request access by sending an email to compassionateuse@avmbiotech.com.
About AVM0703:
AVM0703 is small molecule immunomodulatory drug enrolling Phase 2 trials in US in relapsed/refractory Non-Hodgkin's Lymphoma (NHL) which began enrollment Q3 2023 (partially funded by NCI Ph II FastTrak grant 1R44CA272096). AVM0703 mobilizes a novel endogenous bispecific gamma delta TCR+ invariant TCR+ Natural Killer T-like cell with profound antitumor activity. AVM0703 has shown an absence of safety concerns with side-effects limited to grades 1-3. Clinical responses in the enrolling NHL trial and in FDA-approved expanded access/compassionate use include multiple NHL sub-types and diverse solid tumor types. Responses to AVM0703 are quite rapid, reported from 30 minutes to 14 days after infusion. Preclinical data also demonstrates a significant response against autoreactive lymphocytes in the NOD Type 1 diabetes model (Funded by NIDDK SBIR Ph I grant 1R43DK121634 and NIDDK SBIR Ph II grant 2R44DK121634). Gamma delta TCR+ lymphocytes recognize phosphoantigens expressed by stressed, cancer and infected cells and autoreactive lymphocytes, but not normal cells. Adoptive transfer of AVM0703 induced gamma delta TCR+ immune cells has potent activity against preclinical melanoma (funded by NCI SBIR Ph I grant 1R43CA246896). Additionally, AVM0703 has been shown to have potent neo-adjuvant activity before chemotherapy against immune-resistant, aggressive mouse A20 lymphoma (Funded by NCI SBIR Ph I 1R43_CA271951). Based on its ability to penetrate collagen-encased desmoplastic tumors, AVM0703 has promise as a neoadjuvant prior to chemoimmunotherapy to improve outcomes for metastatic advanced pancreatic cancer patients.
About AVM Biotechnology Inc:
AVM Biotechnology is a clinical stage company headquartered in Seattle, WA developing immunomodulatory therapies for hematological cancer, infectious and autoimmune diseases. AVM’s pipeline includes a small molecule that triggers blood cell formation to replace transfusions, and an allogeneic γδTCR+invTCR+ NKT-like cell. AVM Biotechnology has been selected after competitive review by the National Cancer Institute (NCI) as one of the showcase companies for the 2023-2024 National Cancer Institute Small Business Innovation Research (SBIR) Investor Initiatives. AVM Biotechnology has been awarded five (5) highly competitive National Institute of Health SBIR grants; 2 Phase 1 grants and 1 Phase 2 grant from NCI, and 1 Phase 1 and 1 Phase 2 grant from NIDDK to prevent and reverse type 1 diabetes.
About US Expanded Access Programs:
Sometimes called "compassionate use", expanded access is a potential pathway for a patient with a serious or immediately life-threatening disease or condition to gain access to an investigational medical product (drug, biologic, or medical device) for treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available. Expanded access may be appropriate when all the following apply:
- Patient has a serious or immediately life-threatening disease or condition.
- There is no comparable or satisfactory alternative therapy to diagnose, monitor, or treat the disease or condition.
- Patient enrollment in a clinical trial is not possible.
- Potential patient benefit justifies the potential risks of treatment.
- Providing the investigational medical product will not interfere with investigational trials that could support a medical product's development or marketing approval for the treatment indication.
Investigational drugs, biologics or medical devices have not yet been approved or cleared by FDA and FDA has not found these products to be safe and effective for their specific use. Furthermore, the investigational medical product may, or may not, be effective in the treatment of the condition, and use of the product may cause unexpected serious side effects.
About Compassionate Use Programs:
In New Zealand, prescribers may be aware that Section 25 of the Medicines Act 1981 permits practitioners to procure for sale or supply any medicine for a particular patient in their care. In addition, Section 29 of the Medicines Act 1981 enables a New Zealand company to obtain and supply an unapproved medicine when authorised by a prescriber.
It is the prescriber's responsibility to ensure that they remain cognisant of any safety issues relating to any unapproved medicines they may be prescribing and communicate the risks and benefits to their patients. Medsafe has a number of links on its website that healthcare professionals may find helpful. These links can be found at: www.medsafe.govt.nz/profs/RIss/unapp.asp
Forward Looking Statements: This contains certain statements that constitute "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These statements do not relate strictly to historical or current facts and they may be accompanied by words such as "could," "would," "may," "potentially," "suggest," "believes," "expects," "should," and similar words or expressions. These forward-looking statements reflect our current views as of the date this is published, and are subject to risks, uncertainties, assumptions, changes in circumstances, and other factors; drug development and commercialization are highly risky and early clinical results in animals or humans may not reflect the full results from later stage or larger scale clinical trials. These forward-looking statements are subject to risks and uncertainties that could cause our actual results, performance, and expectations to differ materially from those expressed or implied by these statements, including statements about: future and ongoing drug development and timing; the applications of drugs to specific diseases; the potential for ongoing preclinical or clinical trial results; FDA or other regulatory findings and approvals; potential market opportunities; and the occurrence of future events or circumstances. There are risks and uncertainties involving and not limited to our ability to progress in our research and development efforts, complete clinical testing, achieve our expected results, commercialize our products, avoid infringement of patents, trademarks and other proprietary rights of third parties, protect products from competition, navigate the political environment, maintain sufficient capital and funding, avoid problems with our manufacturing processes, maintain our operations, and obtain regulatory approval to sell and market the drugs in the United States and elsewhere. The reader should not place any undue reliance on such forward-looking statements. We have no obligation to release publicly the results of any revisions to any of our forward-looking statements to reflect events or circumstances after the date these statements are made or to reflect the occurrence of unanticipated events, except as may be required by law.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230731194052/en/
CONTACT: AVM BiotechnologyTheresa Deisher
tdeisher@avmbiotech.comCompassionate Use
Mia Lor
mlor@avmbiotech.comPartnering/Investing
Todd Bertsch
tbertsch@avmbiotech.com
KEYWORD: WASHINGTON UNITED STATES NORTH AMERICA
INDUSTRY KEYWORD: ONCOLOGY HEALTH CONSUMER SENIORS CLINICAL TRIALS PHARMACEUTICAL BIOTECHNOLOGY
SOURCE: AVM Biotechnology
Copyright Business Wire 2023.
PUB: 08/01/2023 10:15 AM/DISC: 08/01/2023 10:16 AM
http://www.businesswire.com/news/home/20230731194052/en