By Julie Steenhuysen
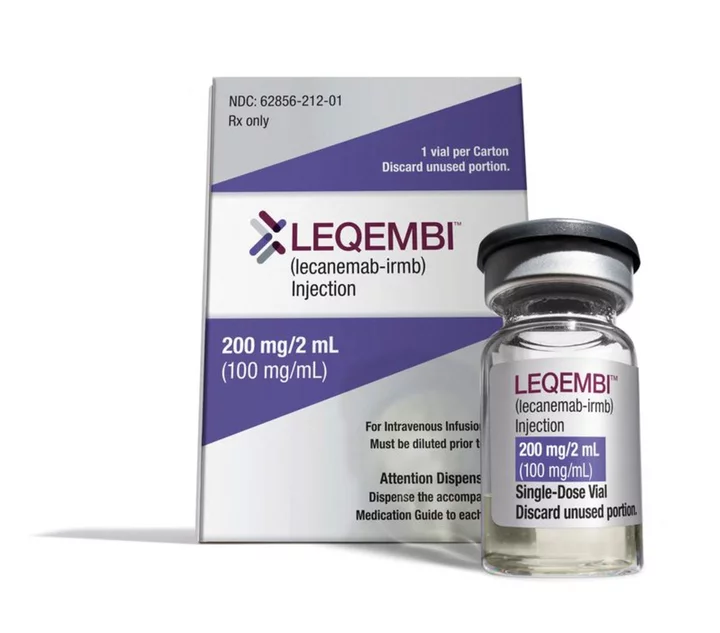
CHICAGO (Reuters) -The U.S. Medicare health plan on Thursday offered details of plans to collect patient data as a condition for reimbursement for Eisai Co Ltd and Biogen Inc's new Alzheimer's drug Leqembi, should it win traditional U.S. approval as expected by July 6.
Under the plan, Medicare, the government health plan for Americans 65 and older, would require physicians to take part in a data collection effort, known as a patient registry, that would be run by the Centers for Medicare and Medicaid Services (CMS).
The nationwide registry, which can be accessed on the CMS website, would be free to use, according to a fact sheet released on Thursday.
If it wins traditional FDA approval, Leqembi would be the first disease-modifying drug ever to achieve the regulatory milestone. Current treatments only treat symptoms but do not change the course of the disease, which affects 6 million Americans, according to the Alzheimer's Association.
To receive reimbursement, doctors will be required to submit demographic information on the provider and patient, the patient's diagnosis, and whether the patient is taking any drugs to treat or prevent blood clots, which may increase the risk of bleeding in the brain associated with the treatment.
The agency also wants to collect data on side effects such as brain swelling or hemorrhages, as well as information on cognitive tests used to assess the patient and any prior treatment.
CMS will also require doctors to submit results of brain scans, spinal fluid tests or other measures used to assess levels of amyloid - an Alzheimer's protein that is the target of Leqembi and other anti-amyloid drugs.
Eisai's Leqembi received unanimous backing by a panel of outside experts to the U.S. Food and Drug Administration earlier this month, who said the company's late-stage trial verified the drug's benefit for patients with early-stage disease.
That study, published in November, showed the drug slowedcognitive decline by 27% in early Alzheimer's patients, but wasalso associated with some serious side effects, including brain swelling and bleeding or microhemorrhages.
Leqembi received accelerated approval in January based on evidence that the drug removed amyloid from the brain. On that basis, CMS said it would only pay for Leqembi if patients were enrolled in a clinical trial.The CMS data requirements apply to all Alzheimer's treatments that reduce beta amyloid from the brain, including Eli Lilly and Co's donanemab, which recently reported positive results from its late-stage trial.
Patient advocates have expressed concern that the registry requirement will limit access to the drug, which is given intravenously and has a U.S. list price of $26,500 per year.
“Today's release from CMS does not provide many new details that are important for physicians, health systems and payers to be ready for delivering coverage upon FDA traditional approval," said Robert Egge, the Alzheimer’s Association chief public policy officer, said in an emailed statement.
"We continue to look forward to CMS providing additional details about their registry and how they will ensure expanded access to FDA traditionally approved Alzheimer’s treatments.”
In its fact sheet, CMS said the agency is "carefully balancing the need to collect information while keeping the registry as easy to use as possible."
CMS said it is working with multiple organizations that are establishing their own registries, and once more are available, doctors will be able to choose which one in which to participate.
(Reporting by Julie Steenhuysen; Editing by Daniel Wallis)