MONROE TOWNSHIP, N.J. & OXFORD, England--(BUSINESS WIRE)--May 30, 2023--
Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals, today announced U.S. Food and Drug Administration (FDA) approval for its optimized, high-affinity radiohybrid (rh) Prostate-Specific Membrane Antigen (PSMA)-targeted PET imaging agent, POSLUMA ® (flotufolastat F 18) injection (formerly referred to as 18 F-rhPSMA-7.3). POSLUMA is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level. It is the first and only FDA-approved, PSMA-targeted imaging agent developed with proprietary radiohybrid (rh) technology. POSLUMA will be commercially available in early June 2023, through certain radiopharmacies in the national radiopharmacy network of Blue Earth Diagnostics’ commercial U.S. manufacturer and distributor, PETNET Solutions Inc, A Siemens Healthineers Company. It will become increasingly available nationally in coming months.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230530005180/en/
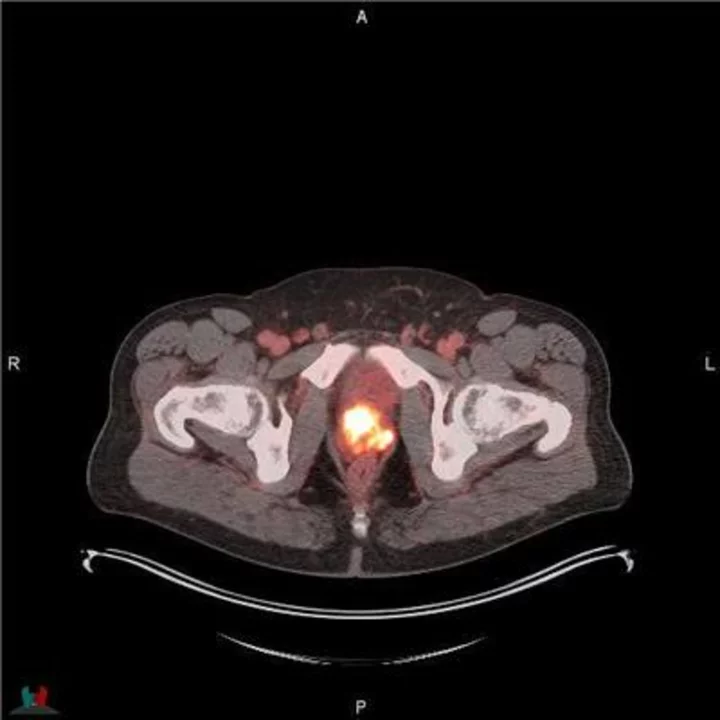
POSLUMA® (flotufolastat F 18) PET/CT image showing uptake in the prostate gland, consistent with primary prostate cancer Photo courtesy of Blue Earth Diagnostics
“With the FDA approval of POSLUMA, we realize our goal of providing an important product that will be widely available across the United States to help inform the management and treatment of patients across the prostate cancer care continuum,” said David E. Gauden, D.Phil., Chief Executive Officer of Blue Earth Diagnostics. “This event marks a major milestone in the expansion of Blue Earth Diagnostics’ robust prostate cancer portfolio. POSLUMA was developed to assist physicians in the detection and localization of prostate cancer. It represents a new class of purposely engineered, high-affinity PSMA-targeted radiopharmaceuticals based on novel radiohybrid technology, which may offer diagnostic imaging and therapeutic potential. All of us at Blue Earth Diagnostics want to express our sincere gratitude to the many patients, physicians, clinical trial sites and collaborators who have worked closely with us to progress POSLUMA.”
POSLUMA is an optimized PSMA-targeted molecule that binds to and is internalized by cells that express PSMA, including prostate cancer cells, which usually overexpress PSMA. It is labeled with the radioisotope fluorine-18 ( 18 F) to enable PET imaging of the prostate and other areas of the body where prostate cancer may have spread. The 18 F radioisotope leverages the high image quality of 18 F-labeled PSMA PET imaging to facilitate effective detection of disease and enables broad, readily available geographic access for patients.
The approval of POSLUMA is based on data from two Blue Earth Diagnostics-sponsored Phase 3 trials (LIGHTHOUSE and SPOTLIGHT), designed to establish the safety and diagnostic performance of POSLUMA across the continuum of prostate cancer care. Results from the LIGHTHOUSE study demonstrated high specificity for the detection of pelvic lymph nodes as compared to histopathology standard of truth in men with PSMA-positive lesions prior to radical prostatectomy. The SPOTLIGHT study evaluated POSLUMA in men with suspected prostate cancer recurrence based on elevated PSA following prior therapy. Results demonstrated high detection rates (% positive PET scans) even at low PSA levels.
In clinical trials, the safety of POSLUMA was evaluated in 747 patients with initial or recurrent prostate cancer. The adverse reactions reported in ≥0.4% of patients in clinical studies were diarrhea, blood pressure increase and injection site pain.
“Effective staging in newly diagnosed prostate cancer – determining its presence and helping determine whether it may have metastasized − is critical in establishing optimal clinical management strategies, because up to 25% of patients with primary prostate cancer may have detectable regional pelvic lymph node metastases, which are correlated with a risk for recurrence and associated overall survival,” said Brian F. Chapin, MD, Associate Professor, Department of Urology, Division of Surgery, The University of Texas MD Anderson Cancer Center, and Coordinating Investigator of the Phase 3 LIGHTHOUSE study. “Conventional imaging techniques such as CT and MRI are limited in the information they may provide. The LIGHTHOUSE study looked at unfavorable intermediate, high and very high risk patients who were scheduled for radical prostatectomy plus pelvic lymph node dissection (PLND) prior to POSLUMA PET. The study showed that POSLUMA PET provided clinically valuable information prior to surgery that would likely result in management changes for these patients.”
“The highly variable nature of recurrent prostate cancer presents clinical challenges, and up to 40% of patients who undergo radical prostatectomy, and up to 50% of patients who undergo radiation therapy will develop local or distant recurrences within 10 years,” said David M. Schuster, MD, FACR, Emory University School of Medicine, Professor of Radiology and Imaging Sciences at Emory University School of Medicine, a researcher at Winship Cancer Institute of Emory University and Coordinating Investigator for the Phase 3 SPOTLIGHT study. “The ability to determine the extent and location of recurrent disease is necessary to inform physicians and their patients for appropriate clinical management. The Phase 3 SPOTLIGHT study investigated the diagnostic performance of POSLUMA PET imaging as a potential decision-making aid in assessing suspected biochemical recurrence of the disease, and demonstrated that it offered precision diagnostic performance even at low PSA levels with an overall 83% detection rate.”
“We at ZERO Prostate Cancer are thrilled to see the approval of POSLUMA as an additional useful tool for staging prostate cancer,” said Shelby Moneer, MS, CHES, Vice President Advocacy and Patient Support, ZERO Prostate Cancer, a patient advocacy organization. “Determining if, when, or where prostate cancer has returned or spread is of the utmost importance for patients and their medical teams. The more patients know about their own diagnosis, the more empowered they are to seek personalized treatment plans. This new approval will, ultimately, give more options – and hope – to people impacted by prostate cancer.”
About POSLUMA ® (flotufolastat F 18)
POSLUMA ® (flotufolastat F 18) injection (formerly referred to as 18 F-rhPSMA-7.3) is an optimized, targeted radiohybrid diagnostic imaging agent indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level. Precision PET imaging with POSLUMA can help identify the location and extent of prostate cancer, providing clinically valuable information to guide patient management. POSLUMA represents a new class of high-affinity PSMA-targeted PET radiopharmaceuticals based on novel radiohybrid technology and is labeled with the radioisotope 18 F to provide readily available patient access and leverage the high image quality of 18 F-labeled PSMA PET imaging to facilitate effective detection of disease. POSLUMA was approved by the U.S. Food and Drug Administration in May, 2023.
About Radiohybrid Prostate-Specific Membrane Antigen (rhPSMA)
Radiohybrid Prostate-Specific Membrane Antigen (rhPSMA) compounds consist of a radiohybrid (“rh”) Prostate-Specific Membrane Antigen-targeted receptor ligand which attaches to and is internalized by prostate cancer cells, and they may be radiolabeled with imaging isotopes for PET imaging, or with therapeutic isotopes for therapeutic use – providing the potential for creating a true theranostic technology. Radiohybrid technology and rhPSMA originated from the Technical University of Munich, Germany. Blue Earth Diagnostics acquired exclusive, worldwide rights to rhPSMA diagnostic imaging technology from Scintomics GmbH in 2018, and therapeutic rights in 2020, and sublicensed the therapeutic application to its sister company Blue Earth Therapeutics. Blue Earth Diagnostics received U.S. Food and Drug Administration approval for its radiohybrid PET diagnostic imaging product for use in prostate cancer in 2023. rhPSMA compounds for potential therapeutic use are investigational and have not received regulatory approval.
Indication and Important Safety Information About POSLUMA
INDICATION
POSLUMA ® (flotufolastat F 18) injection is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer
- with suspected metastasis who are candidates for initial definitive therapy
- with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level
IMPORTANT SAFETY INFORMATION
- Image interpretation errors can occur with POSLUMA PET. A negative image does not rule out the presence of prostate cancer and a positive image does not confirm the presence of prostate cancer. The performance of POSLUMA for imaging metastatic pelvic lymph nodes in patients prior to initial definitive therapy seems to be affected by serum PSA levels and risk grouping. The performance of POSLUMA for imaging patients with biochemical evidence of recurrence of prostate cancer seems to be affected by serum PSA levels. Flotufolastat F 18 uptake is not specific for prostate cancer and may occur in other types of cancer, in non-malignant processes, and in normal tissues. Clinical correlation, which may include histopathological evaluation, is recommended.
- Risk of Image Misinterpretation in Patients with Suspected Prostate Cancer Recurrence: The interpretation of POSLUMA PET may differ depending on imaging readers, particularly in the prostate/prostate bed region. Because of the associated risk of false positive interpretation, consider multidisciplinary consultation and histopathological confirmation when clinical decision-making hinges on flotufolastat F 18 uptake only in the prostate/prostate bed region or only on uptake interpreted as borderline.
- POSLUMA use contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer. Advise patients to hydrate before and after administration and to void frequently after administration. Ensure safe handling to minimize radiation exposure to the patient and health care providers.
- The adverse reactions reported in ≥0.4% of patients in clinical studies were diarrhea, blood pressure increase and injection site pain.
- Drug Interactions: androgen deprivation therapy (ADT) and other therapies targeting the androgen pathway, such as androgen receptor antagonists, may result in changes in uptake of flotufolastat F 18 in prostate cancer. The effect of these therapies on performance of POSLUMA PET has not been established.
To report suspected adverse reactions to POSLUMA, call 1-844-POSLUMA (1-844-767-5862) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Full POSLUMA prescribing information is available atwww.posluma.com/prescribing-information.pdf.
About Blue Earth Diagnostics
Blue Earth Diagnostics, an indirect subsidiary of Bracco Imaging S.p.A., is a growing international molecular imaging company focused on delivering innovative, well-differentiated diagnostic solutions that inform patient care. Formed in 2014, the Company’s success is driven by its management expertise and supported by a demonstrated track record of rapid development and commercialization of positron emission tomography (PET) radiopharmaceuticals. Blue Earth Diagnostics’ expanding oncology portfolio encompasses a variety of disease states, including prostate cancer and neuro-oncology. Blue Earth Diagnostics is committed to the timely development and commercialization of precision radiopharmaceuticals for potential use in imaging and therapy. For more information, please visit: www.blueearthdiagnostics.com.
About Bracco Imaging
Bracco Imaging S.p.A., part of the Bracco Group, is a world-leading diagnostic imaging provider. Headquartered in Milan, Italy, Bracco Imaging develops, manufactures and markets diagnostic imaging agents and solutions. It offers a product and solution portfolio for all key diagnostic imaging modalities: X-ray imaging (including Computed Tomography-CT, Interventional Radiology, and Cardiac Catheterization), Magnetic Resonance Imaging (MRI), Contrast Enhanced Ultrasound (CEUS), and Nuclear Medicine through radioactive tracers and novel PET imaging agents to inform clinical management and guide care for cancer patients in areas of unmet medical need. Our continually evolving portfolio is completed by a range of medical devices, advanced administration systems and dose-management software. In 2019 Bracco Imaging enriched its product portfolio by expanding the range of oncology nuclear imaging solutions in the urology segment and other specialties with the acquisition of Blue Earth Diagnostics. In 2021, Bracco Imaging established Blue Earth Therapeutics as a separate, cutting-edge biotechnology vehicle to develop radiopharmaceutical therapies. Visit: www.braccoimaging.com.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230530005180/en/
CONTACT: For Blue Earth Diagnostics (U.S.)
Priscilla Harlan
Vice President, Corporate Communications
(M) (781) 799-7917
priscilla.harlan@blueearthdx.comFor Blue Earth Diagnostics (UK)
Clare Gidley
Associate Director Marketing and Communications
Tel: +44 (0) 7917 536939
clare.gidley@blueearthdx.comMedia
Sam Brown Inc.
Mike Beyer
(M) (312) 961-2502
mikebeyer@sambrown.com
KEYWORD: EUROPE UNITED STATES UNITED KINGDOM NORTH AMERICA NEW JERSEY
INDUSTRY KEYWORD: SCIENCE RADIOLOGY BIOTECHNOLOGY RESEARCH ONCOLOGY HEALTH FDA CLINICAL TRIALS
SOURCE: Blue Earth Diagnostics
Copyright Business Wire 2023.
PUB: 05/30/2023 07:00 AM/DISC: 05/30/2023 07:00 AM
http://www.businesswire.com/news/home/20230530005180/en